

Document Type : Original Article
Authors
1 Department of Zoology at the University of Benghazi, Benghazi, Libya
2 Plant Protection Department at Omar Al-Mukhtar University, Al Bayda, Libya
Abstract
Keywords
Honey is the substance produced by honey bees after collecting plants’ nectar and sugary secretions, where it undergoes a series of enzymatic reactions before being regurgitated and stored in the hexagonal wax cells. The chemical composition of honey is complex and rich. Honey contains various sugars, especially monosaccharides such as glucose and fructose. Additionally, it contains different levels of proteins, amino acids, enzymes, organic acids, and mineral substances based on its source [1]. Due to its high nutritional value and attractive flavor, honey is considered a valuable component in the human diet [1].
Honey has the ability to fight food-borne pathogens such as Escherichia coli, salmonella, and other bacteria, including Staphylococcus aureus and Pseudomonas aeruginosa [2] with many reports of its antimicrobial and antifungal activities [3][4]. This activity might be attributed to its osmotic pressure; the low pH, which ranges from 3.2 to 4.5 [5]; hydrogen peroxide content; the antimicrobial peptide defensin-1, which is synthesized in the bee’s salivary glands [6]; as well as the presence of phytochemical agents [3][4]. Based on its original source, honey showed antibacterial activity, especially against wound infections and inflammation [7]. With antibiotic resistance becoming a serious issue nowadays [8], honey is being rediscovered as an effective wound care product [9]. Therefore, honey is considered a convenient antimicrobial therapy due to its relatively low cost and local availability [10].
Medicinal plants are the oldest sources of pharmacologically active compounds. Although synthetic pharmaceuticals are widely produced and consumed, medicinal plants continue to meet the therapeutic needs to play a dominant role in introducing new therapeutic agents. Caper (Capparis spinosa L.) is known for its biologically active compounds, especially polyphenols, and it has been used for centuries in Indian traditional medicine as a medicine for various human diseases [11]. Caper honey is a dark brown color, slow-freezing honey reported to increase immunity experimentally [12].
Considering the previously mentioned points, this study aims to:
1. Investigating the chemical composition of Capparis spinosa honey.
2. Investigating capris honey antibacterial effect against Staphylococcus aureus and Escherichia coli.
Caper (Capparis spinosa L.) honey was sampled from the middle of May to the middle of September.
This study concentrated on two bacterial species, Escherichia coli and Staphylococcus aureus since these bacteria are responsible for various infections such as urinary tract infection (UTI) and Post-operative wound infection. The bacterial strains were obtained from Ibin-Zahr laboratory in the form of 24-hour-old bacterial broth cultures in sterilized glass bottles with tight lids. The bacterial samples were kept at 4 °C until secondary culturing before the experiment.
Total dissolved solids were measured using a refractometer at a temperature of 20 °C. From the refractive index readings, the percentage of water content in honey samples was estimated using the relationship between refractive index and water content [13].
The pH of honey was measured using a pH meter (Wissenenschagl-Tech, Werstaten, Wtw, Microprocessor pH meter) [13].
Determination of total sugars, vitamins, and protein content in honey samples was performed using (UV) method. For this purpose, 10 g of well-mixed honey was placed in a glass container and heated for 15 minutes at a temperature of 60 °C, with constant stirring. Honey samples were then left to cool down. 1 g the treated honey samples were weighed and placed in a volumetric flask and diluted with distilled water to 100 ml. 0.1 ml of the final solution was used to estimate the percentage of total sugars and proteins at wavelengths of 490 and 510 nm, respectively, according to [14][15] using a UV/VIS Spectrophotometer (Thermo Fisher 8735).
Calcium, potassium, and phosphorous contents were estimated in honey samples using Electrolyte Analyzer (A&T 980 / Biomedical Instrument, Switzerland).[16]
This test was carried out by dipping discs of sterile filter paper in sequential concentrations of caper honey solution [0 (Control), 15, 25, 50, 75%] (w:v) diluted using sterile distilled water. These discs were placed using sterile forceps on the centers of Petri dishes, which were previously filled with nutrient agar and inoculated with the studied bacteria. Three replicates (dishes) were prepared for each honey concentration, and the dishes were then incubated at 37 °C. After 24 hours, the diameter of the inhibition area around each disc was measured in millimeters.
This test was carried out using the same caper honey concentrations in the growth inhibition experiment [0 (Control), 15, 25, 50, 75%] (w:v) diluted in sterile normal saline. The bacterial suspensions were prepared by scraping an individual colony from the original culture and adding it to 10 ml of sterile normal saline, from which, serial dilutions were prepared by adding 1 ml of the original suspension to 9 ml of sterile normal saline and repeat this procedure until reaching a concentration of 10-5. 1 ml of the final dilution was added to a test tube containing 9 ml of Honey solution with the required concentration. Three tubes were used as replications for each concentration. The tubes were incubated for 12 hours at a temperature of 37 °C. After that, 1 ml of the honey solution containing the bacterial suspension was added to a Petri dish containing the nutrient agar medium, and the dishes were incubated for 24 hours at a temperature of 37 °C. The dishes were removed from the incubator, and the growing bacterial colonies were counted using a counter. The bacterial counts were represented in colony-forming unit per ml (Cfu/ml).
The results were submitted to analysis of variance (ANOVA) and means were compared using Tukey’s test (p ≤ 0.05) with Minitab software.
The results showed that caper honey total solids content was 81.1%, and therefore, water content was 18.9%. The high moisture content is due to the high moisture content of caper flowers nectar. Additionally, the harvest season also influences honey moisture content as fall and winter honey has a higher water content than that collected in the spring and summer seasons. High moisture levels can result from poor harvesting practices, or they can indicate early harvest, which may occur when honey is immature [1]. According to the current legislation, the maximum allowable moisture content in honey is 20%. Sugar content reached 74.30%; while on the other hand, calcium, potassium, and phosphorus contents were 0.07, 0.15, and 0.02 %, respectively. Protein content was 0.83%; however, these proteins include several enzymes in small quantities and significant antibacterial activities. Therefore, this modest protein content is considered highly beneficial from a medical and nutritional point of view. The recorded pH was 4.8 (Table 1).
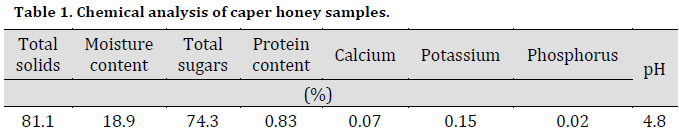
The disk diffusion experiment showed that all caper honey dilution had significantly inhibited bacterial growth compared to control treatment (Fig. 1 A). Increasing caper honey concentration was accompanied by a persistent increase in growth inhibition zone diameter for both bacterial species with 28 and 24 mm in E. coli and S. aureus, respectively, using 75% honey concentration. However, this increase was significant until 50%, with no significant differences between 50% and 75% treatments in both bacterial inhibition zone diameters. Interestingly, the inhibition effect was more pronounced against E. coli; however, E. coli inhibition zone diameter was significantly higher than that of S. aureus only under 50% treatment.
The effect of Caper honey on the numbers of study bacteria S. aureus and E. coli was investigated using plate count methods. A steady decrease in the bacterial count for both species was noticed by increasing honey concentration. The number of E. coli and S. aureus colonies decreased from 225 and 120 Cfu/ml, respectively, in control to 0 Cfu/ml using 75% caper honey concentration treatment. However, no significant decrease in E. coli and S. aureus bacterial counts after 25% and 50% treatments, respectively (Fig. 1 B).
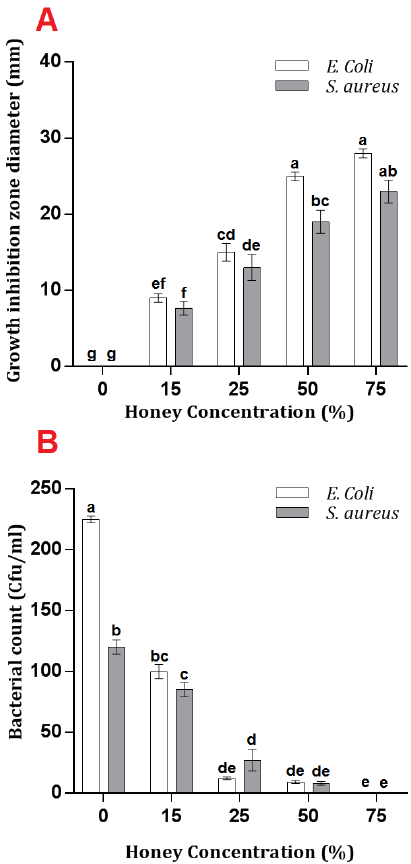
The results of this study agreed with the results of [17][18][19], where an increased honey concentration resulted in an increase in bacterial inhibition one diameter. The results were also in correspondence with [20], who reported various inhibition degrees of E. coli and Pseudomonas aeruginosa under 5%, 10%, 20% honey concentrations. Other studies also reported the bacterial inhibitory effects of wild honey [21][22][23]. However, the current study suggested 50% and 75% caper honey concentrations as sufficient potential bacterial inhibitors under the studied conditions.
The current study nominates caper honey as an effective antibacterial agent comparable to commercial antibiotics since similar results were obtained previously by [24] using Uremia honey, and [25] using types of honey from India and New Zealand. Both studies obtained inhibition results against the growth of Gram-negative and Gram-positive bacteria similar to that of standard antibiotics.
Conflict of interest statement
The authors declared no conflict of interest.
Funding statement
The authors declared that no funding was received in relation to this manuscript.
Data availability statement
The authors declared that all related data are included in the article.