

Document Type : Original Article
Authors
1 Department of Anatomy and Histology, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Bangladesh
2 Animal Production Division, Department of Livestock Services, Government of People’s Republic of Bangladesh, Dhaka, Bangladesh
3 Bangladesh Livestock Research Institute, Savar, Dhaka, Bangladesh
4 Bangladesh Agricultural Research Council, Farmgate, Dhaka, Bangladesh
5 Department of Pathology, Faculty of Veterinary Science, Bangladesh Agricultural University, Bangladesh
Abstract
Keywords
Vero cell lines (Vero, Vero 76, and Vero E6) are derived from African green monkey [1]. These lines are used primarily in the virological sector, where the maintenance of frozen stocks is essential to discontinue regular subculturing. This preservation practice is essential to cut expenses, save time, and prevent infection/contamination incidents. Preservation also brings a new source of cells during subsequent passages [2][3].
Cryobiology investigates low-temperature effects on living organisms, tissues, or cells, and Cryopreservation is the most commonly used method that allows low-temperature maintenance of a variety of tissues/cells [4]. Revivability and viability of preserved cells are among the major concerns of cryobiology studies. These two terminologies are associated with various factors, including the cryoprotectant properties such as its concentration and mode of action, freezing and thawing method, cell density, preservation temperature, and duration. Besides cryopreservation in liquid nitrogen temperature range (-140°C to -196°C), deep freezing (-80°C) is widely used to preserve biological materials. Although cryopreservation is recommended in cryo studies, maintaining liquid nitrogen is more expensive compared to deep freezing. As for cryoprotectants, Dimethyl sulfoxide (DMSO) and glycerol are widely used. glycerol is cheaper and more available; however, the data available regarding its optimum concentration in preservation medium is scarce.
The used freezing technique prior to cold preservation is a determinant factor in the success of cell preservation. The slow freezing protocol reported by [5] resulted in excellent post-preservation observations. Therefore, this study was directed to investigate the impact of different preservation variables, including cryoprotectant type and concentration, freezing method, and preservation temperature on the post-thawing viability of preserved Vero cells.
frozen stock Vero cells [6][7][8] were used in this study. Vero cells were obtained from cell line service (CLS, order no. 605372, Germany) (Fig. 1 A).
The media and reagents used in the current study were: M-199 (Gibco-Invitrogen, cat no. 11825), Bovine calf serum (Gibco-Invitrogen, cat no.10437), 0.25% Trypsin with EDTA (Gibco-Life technologies 20367,C13), DMSO (Gibco-Invitrogen) and glycerol.
Continuous Vero cells sub-culturing and maintenance were accomplished as recommended by [5]. In brief, when confluence reached 80%–100%, the medium was expelled. After washing with PBS, cell dissociation by trypsinization was performed (1 ml of 0.25% Trypsin-EDTA solution). Trypsinization was terminated by adding 1 mL of 50% calf serum. After 10 min of centrifugation at 3000 rpm, the supernatant was poured, and the precipitated cells were resuspended with M-199 medium with 10% calf serum and 2% penicillin-streptomycin solution. Seven successive subcultures were completed to obtain a healthy and strong pure cell for use in the study,
Vero cells (80%–90% confluence) were rinsed and dissociated with Trypsin-EDTA solution. Quick microscopic quality control was conducted at 37°C under an inverted phase contrast microscope (Olympus, CK2-TR, Japan). Then, 10% calf serum was added to terminate trypsinization. The suspension was centrifuged for 10 min at 3000 rpm, and the supernatant was poured. The harvested cells were resuspended with cell culture medium, calf serum, and antibiotics solution with freezing medium containing dimethyl sulfoxide (DMSO) (5 or 10%) or glycerol (5 or 10%) and made up to 100% with a final concentration of 106 – 3×106 viable cells per mL. The resuspended cells were distributed into labeled 2.0 ml cryogenic vials (Corning).
Properly labeled cryovials were introduced to a scheduled temperature gradient. First, the vials were kept at 2-8°C for 2 hours, then -20°C for 6 hours, followed by -80°C overnight in a deep freezer. The cryovials were finally cryopreserved at liquid nitrogen (-196°C) (Cryopreservation treatment) in liquid nitrogen or kept at deep freezer (Deep freezing treatment).
Properly labeled cryovials were transferred directly to the deep freezer (-80°C) or liquid nitrogen (-196°C).
Both slowly and rapidly frozen cryovials were preserved under these constant conditions for 18 months.
An equal number of the preserved Vero cells (30 vials from each treatment) were used for the revivability test providing the same environment and culture media for continuous sub-culturing following [5] recommendations. The vials were thawed in a water bath at 37°C. Trypan blue dye exclusion staining method [9] was performed to estimate post-thawing cell viability. An equal volume of the thawed cell stock and 0.4% trypan blue dye solution were mixed instantly after thawing. Cell suspension (20 µl) was examined using a hemocytometer (Fig. 1 B). Based on the development of blue color, dead and viable Vero cells were counted (Fig. 1 C), and viability was estimated using the following formula:
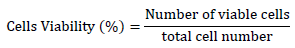
Revivability was recorded for each vial as 0 (not-revived) or 1 (revived). In order to compare the investigated treatments in terms of revivability, the binary logistic regression method was. Viability test means were compared using ANOVA test. All statistical analyses were conducted using Minitab 19.
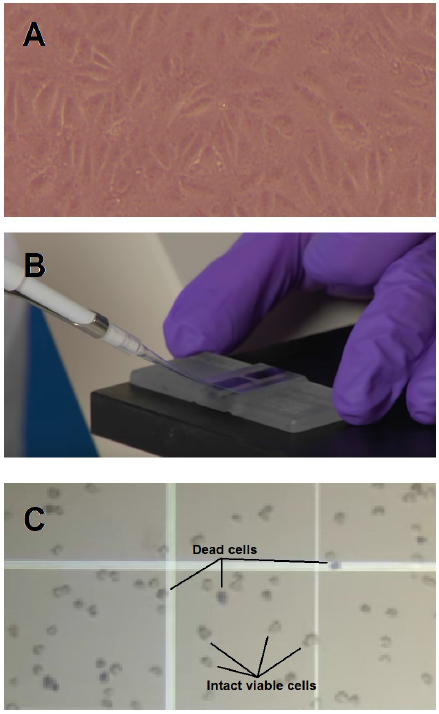
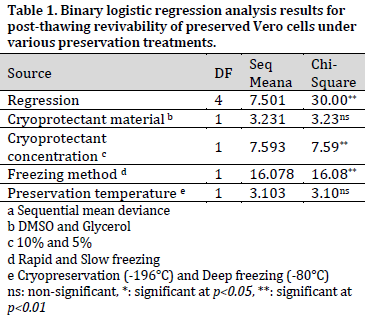
The highest revivability was recorded in the event of 10% glycerol with combined with slow freezing method and cryopreservation conditions (83.33%) while the lowest revivability was observed under 5% DMSO combined with rapid freezing method and a deep freezing preservation (Fig. 2 A). Overall, it was noticed that glycerol, higher cryoprotectant concentration (10%), slow freezing method, and cryopreservation (-196°C) treatments scored higher than DMSO, lower cryoprotectant concentration (5%), rapid freezing method, and deep freezing treatments, respectively (Fig. 2 A). However, binary logistic regression analysis showed that the increase in revivability was only significant when comparing different cryoprotectant concentrations and freezing methods (Table 1). No significant effect of treatment combinations was observed; therefore, the combined effects were not considered in the binary logistic regression model.
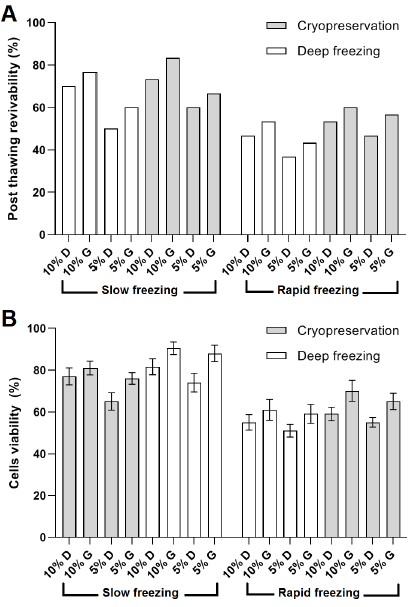
Similar to the revivability test, the highest viability percentage was observed under 10% glycerol treatment combined with slow freezing technique and cryopreservation reaching 90.5%. In contrast, the lowest viability percentages were observed under the combination of using DMSO as a cryoprotectant with rapid freezing regardless of preservation temperature (Fig. 2 B). Significant differences were noticed between freezing methods, with slow freezing being superior compared to the rapid method.
The highest revivability results were observed using the slow freezing method regardless of other variables, similar to the findings reported in [8]. This observation is attributed to the higher risk of sudden freeze shock in the cells exposed to the rapid freezing method compared to the slow freezing method. These findings support the presentment of [6][7]. In fact, it is preferable to decrease storage vials temperature gradually at a rate of 1°C per minute for better preservation results [5], which corresponds with the slow freezing method used in this work. The cells viability was found the highest using the slow freezing method,
It was noticed that glycerol resulted in better preservation results than DMSO. The differences between glycerol and DMSO might be due to the differences in their mode of action as cryoprotectants. However, there were no overall significant differences between the two substances. Therefore, the use of glycerol as a cheap cryoprotectant can be recommended for long-term Vero cells preservation.
On the other hand, a higher cryoprotectant concentration resulted in higher revivability results, which corresponds with [8]. The highest cell viability in the case of DMSO was 81.6% at 10% concentration, which agrees with [10], who recommended the use of 10% DMSO to preserve BHK-21 cells. On the other hand, few related studies have been conducted with glycerol being the recommended cryoprotectant. For instance, [11] reported using 10% glycerol for the cryopreservation of rat morulae. Another study was carried out by [12] on cryopreservation of rhesus monkey (Macaca mulatta) sperm where it was found that 5% glycerol had a better preservative effect compared to 5% DMSO, which is in accordance with the present findings.
Although higher viability was observed under high cryoprotectant concentration, which is similar to many previous reports [8][13][14], toxicity studies revealed that DMSO shows maximum notoxious effect on animal cells above 4% [15], which might negatively affect cell viability. Similarly, glycerol at a concentration of 5% resulted in high toxicity in cryopreserved stallion spermatozoa [16]. Therefore, more research should be carried out to optimize cryoprotectant concentration in the preservation medium.
It can be concluded that using 10% glycerol as cryoprotectant followed by the reported slow freezing protocol and deep freezing or, more preferably, cryopreservation may be a suitable method in cryobiology for the long term Vero cells preservation.
Conflict of interest statement
The authors declared no conflict of interest.
Funding statement
The authors declared that this work was supported by Bangladesh Agricultural Research Council (BARC), Ministry of Agriculture, Government of the Peoples Republic of Bangladesh (Grant numbers SPGR-360, NATPPhase-1, BARC).
Data availability statement
The authors declared that all related data are included in the article.